The Polymer Research Institute was established in 1946 by Herman F. Mark, a pioneer in the study of giant molecules. The Institute brought together a number of polymer researchers to create the first academic facility in the United States devoted to the study and teaching of polymer science. Scientists associated with it later went on to establish polymer programs at other universities and institutions, contributing significantly to the development and growth of what has become a vital branch of chemistry, engineering, and materials science.
ACS



Havemeyer Hall was built between 1896 and 1898 under the leadership of Charles Frederick Chandler. It provided research and teaching facilities for faculty and students specializing in industrial, inorganic, organic, physical, and biological chemistry. Pioneering research done here led to the discovery of deuterium, for which Harold Clayton Urey received the Nobel Prize in 1934. Six others who did research here subsequently received the Nobel Prize, including Irving Langmuir, the first industrial chemist to be so honored, in 1932.

Gilman Hall, built in 1916-1917, accommodated a growing College of Chemistry by providing expanded research and teaching facilities for faculty and students specializing in physical, inorganic and nuclear chemistry. Work performed at Gilman Hall helped advance the fields of chemical thermodynamics and molecular structure, and has resulted in multiple Nobel Prizes. The Hall is most famous for the work of Glenn T. Seaborg and his coworkers, which included the successful identification and production the element Plutonium. Seaborg received the Nobel Prize in 1951 for his accomplishments.
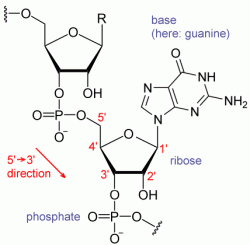
In 1961, in the National Institutes of Health Headquarters (Bethesda, MD), Marshall Nirenberg and Heinrich Matthaei discovered the key to breaking the genetic code when they conducted an experiment using a synthetic RNA chain of multiple units of uracil to instruct a chain of amino acids to add phenylalanine. The uracil (poly-U) served as a messenger directing protein synthesis. This experiment demonstrated that messenger RNA transcribes genetic information from DNA, regulating the assembly of amino acids into complex proteins.
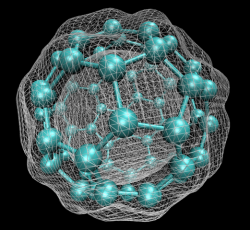
In early September 1985, a team of scientists discovered a previously unknown pure carbon molecule, C60, which they dubbed buckminsterfullerene. The name was chosen because the geodesic domes of Buckminster Fuller provided a clue that the molecule’s atoms might be arranged in the form of a hollow cage. The structure, a truncated icosahedron with 32 faces, 12 pentagonal and 20 hexagonal, has the shape of a soccer ball.

The William H. Chandler Chemistry Laboratory was conceived and planned by William Henry Chandler (1841-1906), professor, chairman, librarian, and acting president of Lehigh University. Designed by Philadelphia architect Addison Hutton and erected between 1884 and 1885 at a cost of $200,000, the building set the standard for laboratory construction for the next half century.
Antoine-Laurent Lavoisier studied at the Académie des Sciences de l'Institut de France (then "Collège Mazarin") from 1754 to 1761. He was elected to the Royal Academy of Sciences in 1768, where he presented his important studies on oxygen in chemistry. These began with a "pli cacheté" of Nov. 2, 1772, and, after he experimentally proved the chemical composition of water by the quantitative method, culminated in his abandoning of the phlogistic theory in 1785.

Around 1907, Belgian-born chemist Leo Hendrik Baekeland took two ordinary chemicals, phenol and formaldehyde, mixed them in a sealed autoclave, and subjected them to heat and pressure.
The sticky, amber-colored resin he produced in his Yonkers laboratory was the first plastic ever to be created entirely from chemicals, and the first material to be made entirely by man.

When Arnold Beckman, a professor of analytical chemistry at the California Institute of Technology, was asked to devise a way to measure acidity in citrus fruit, the resulting “acidometer” revolutionized chemical instrumentation. The innovative features of the pH meter, including its use of integrated electronic technology and all-in-one design, were the basis for subsequent modern instrumentation developed by Beckman and his company.
The plaque commemorating the development reads:

Bread is considered a basic foodstuff; eaten down through the ages, it continues to be a staple of the modern diet. The development of baking powder made baking easier, quicker and more reliable for bakers in the mid-19th century. Eben Horsford’s unique formula was an important innovation and made the making of biscuits, cookies and other quick baking products simpler than before.
The commemorative plaques read:


